David KaslowPATH
Dr. David Kaslow is Vice President of Product Development at PATH, a global nonprofit dedicated to ending health inequity.
In this post, PATH Vice President of Product Development, David Kaslow, writes about the first-ever shipment of semisynthetic artemisinin (ssART)—a new type of malaria treatment that marks a significant milestone in the fight against malaria. This post originally appeared on the PATH blog on August 12.
Malaria drugs made with semisynthetic artemisinin make their way to patients
This month, after nearly ten years of effort, the first batch of malaria drugs manufactured with a new, semisynthetic form of the key ingredient, artemisinin, will start reaching African countries battling the disease. The lifesaving drugs, manufactured by our partner Sanofi, a French pharmaceutical company, are the first of their kind to use ssART in place of the plant-derived form of artemisinin used in the past. The new shipment marks a milestone in global health, potentially improving access to treatment for the millions of people, mostly African children, sickened by malaria every year.
The story of ssART—and the bold partnership that brought it from concept to use—highlights the crucial role international markets play in shaping global health, and the power of collaborative innovation to improve the systems that bring lifesaving products to the people who need them.
Soothing a volatile market
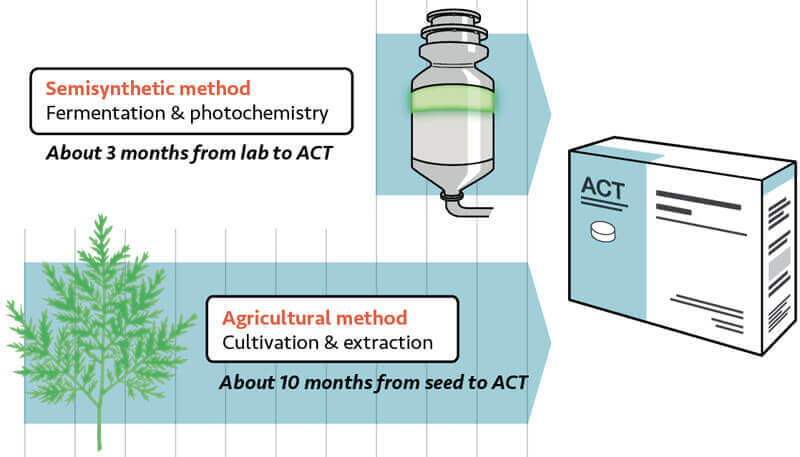
In 2004, doctors worldwide were increasingly using a powerful group of drugs called artemisinin-based combination therapies (ACTs), to treat malaria infection. That was good news for children and families everywhere. But as more countries sought ACTs, the supply and price of artemisinin—which was derived only from a slow-growing plant called Artemisia annua, or Asian sweet wormwood—fluctuated wildly. Changes in demand and supply from year to year created a volatile up-and-down cycle of pricing and supply. In 2005, for example, the price of artemisinin was at a high of US$1,100 per kilogram. That price sparked more farmers to plant sweet wormwood, creating an oversupply in 2007 that caused the price to plummet to just $180 per kilogram. That cut the incentive to grow sweet wormwood again, which resulted in a 2009 shortage that drove prices back up over the next two years, to an average of $530 per kilogram in 2011. These fluctuations strained markets, and made it difficult for leaders, manufacturers, and others to stabilize and plan global supply, risking a global shortage.
In short, the lifesaving drugs that millions of children, families, and communities relied upon were tied to a supply structure struggling under the push and pull of a volatile market. Worldwide, a market challenge had turned into a crucial global health imperative.
A groundbreaking partnership
Solving the challenge took a groundbreaking partnership, led by PATH’s Drug Development program, which brought together experts in research, pharmaceutical product development, and public health. In 2004, the partnership set out to develop a new manufacturing process to produce high-quality, year-round, and affordable artemisinin to supplement the plant-based supply. Over a decade of work, our combined innovation and vision brought a promising concept from small laboratory batches, to large-scale production, and on to global markets.
Now, as the first shipment of ssART-based malaria drugs—1.7 million treatments of Sanofi’s gold-standard ACT, Artesunate Amodiaquine Winthrop®—make their way to customers for the first time, we’ve reached the goal we set out to achieve ten years ago. Semisynthetic artemisinin opens a new future for the global artemisinin market. In doing so, it offers new security for the millions of people who rely on ACTs in countries where malaria is a constant threat. By providing a year-round source, ssART helps to manage imbalances in supply and demand, maintain stable and affordable pricing, and keep the market on an even course—ultimately expanding access to ACTs.
The future

Of course, ssART is only one part of the solution. Eliminating and controlling malaria requires a highly integrated strategy, including new vaccines and diagnostics, expanded surveillance and control, strong partnerships with governments, and much more.
I think I can speak for all of our partners when I say that we won’t be satisfied until that very last patient is cured of malaria. Until then, however, the sustained production of ssART, and the careful integration of our product into global markets, has the potential to change the landscape of global health. For the first time in history, artemisinin shouldn’t be a limiting factor in our work to develop and deploy lifesaving treatments. Even more, ssART provides clear evidence that with expertise, hope, and tenacity, it is possible for public and private sector leaders to achieve a bold humanitarian goal.
Together, we’re paving the way for a robust, reliable supply of antimalarial drugs. And by continuing to work together, we can create a market where supply chains, farmers, extractors, manufacturers, and funders all align to create a healthier future for women and children worldwide.
More Information