
- Wendy Taylor of USAID introducing the other event partners. Photo: Marissa Chmiola/GHTC
Last Wednesday 53 teams of innovators gathered at the International Trade Center in Washington, DC, for the Saving Lives at Birth DevelopmentXChange—an
event resembling a cross between a science fair and an episode of Shark Tank. There were no baking soda volcanoes or smiley sponges to be found,
rather innovative ideas to advance the health of pregnant women and newborns in poor communities around the world. And at stake was not a blue
ribbon or a chance to take home Mr. Wonderful’s money, but something much more important—the chance to save the lives of mothers and their
babies.
Saving lives at birth through innovation
This DevelopmentXChange served as the final stage of competition in the 5th annual Saving Lives at Birth Grand Challenges Program, a contest sponsored
by the US Agency for International Development (USAID), the government of Norway, Grand Challenges Canada, and the UK Department of International
Development, which aims to identify and advance groundbreaking and cost-effective solutions to improve maternal and newborn health. Innovators
from around the world compete for grants to develop, test, and bring their ideas to scale. The competition covers three areas—technology
development, service delivery, and demand creation.
The program started in 2011 as a way to “leapfrog” solutions in the underserved area of research. “We recognized that the area of maternal and neonatal
health is an area that had really not seen the type of innovation we need to save the lives of mothers and newborn around the time of birth and
in the hardest to reach areas,” said Wendy Taylor, director of the Center for Accelerating Innovation and Impact at USAID. “So we put together
the Saving Lives at Birth Grand Challenge…and called on the brightest minds across the world, engineers and entrepreneurs, scientists and
students, to come up with new, groundbreaking ideas, new ways to prevent and treat mothers and newborns and save their lives in the most risky
time—the 48 hours around the time of birth.”
The response to this call has been tremendous. Since 2011, the program has provided over US$47 million in funding to 81 innovative tools and approaches,
and many of the program’s successes are already having an impact as they begin to scale. To date, Saving Lives at Birth–supported innovations have
been delivered to over 1.5 million women and newborns, saving at least 4,000 lives.
This year the Saving Lives at Birth competition received more than 750 submissions, over half of which came from low-and middle-income countries (LMICs).
Fifty-three teams were chosen to compete as finalists at the DevelopmentXChange. At the conclusion of the event, 17 award nominees were selected with additional nominees in the transition-to-scale category to be announced later this year.
GHTC member projects featured as finalists
Among the 53 finalists were 10 projects from GHTC member organizations:
Validation of low-cost, user-friendly strip test for preeclampsia/eclampsia screening
PATH

- Brandon Troy Leader showcases his team's innovation. Photo: GHTC/Courtney Carson
Dr. Brandon Troy Leader and his team at PATH are developing a low-cost, easy-to-use strip to test for proteinuria—a key indicator of preeclampsia/eclampsia—during
pregnancy. Preeclampsia is a serious health risk to both mothers and infants and accounts for at least 16 percent of maternal deaths in low-resource
settings. Current diagnostics are either time-consuming and expensive—requiring 24 hour urine analysis—or inaccurate—relying
on analysis of protein levels in urine which can fluctuate with fluid intake. Both of these tools are impractical for use in secondary health centers,
where most women in LMICs receive treatment. The team’s innovative dipstick promises to deliver a quick, low-cost, and highly accurate test that
will allow more women in low-resource settings to be diagnosed and appropriately monitored for preeclampsia/eclampsia. The strip tests for a new
protein-to-creatinine (PR/CR) marker and uses a product specifically designed for this single application. It is available at less than $0.10 a
strip—the current cost of the inaccurate protein-urine analysis diagnostic. In lab tests, the PR/CR strips showed 85 percent sensitivity,
indicating that with appropriate clinical trials, the product could be quickly rolled out to communities in need. The team was seeking a Saving
Lives at Birth validation award to test the PR/CR strips in a clinical trial with over 20,000 women and accelerate the product’s introduction into
low-resource settings.
Advancing introduction of new screening test for preeclampsia
PATH
To address the unmet need for effective screening and treatment of preeclampsia in low-resource settings, PATH is preparing for a large-scale evaluation
of its innovative glycosylated fibronectin (GlyFN) test. The test targets the GlyFN biomarker, which occurs at higher concentration in the blood
than other preeclampsia biomarkers and offers excellent sensitivity and specificity. Small scale tests indicate that the GlyFN test predicts preeclampsia
in the third trimester of pregnancy with a sensitivity of 97 percent and specificity of 93 percent. The GlyFN test is innovative not only for its
use of an easier to detect biomarker, but also for its accuracy and sensitivity in diagnosis. The PATH team was seeking a Saving Lives at Birth
award to perform the first operational evaluations of the GlyFN test, refine training materials with users in the field, and implement a prospective-cohort
study in Karnataka, India.
Developing a model for national scale-up of the Pratt Pouch to prevent mother-to-child HIV transmission
Elizabeth Glaser Pediatric AIDS Foundation (EGPAF)

- A syringe typically used to measure ARV dosage and the Pratt Pouch which could replace it. Photo: GHTC/Marissa Chmiola
Dr. Edward Bitarakwate and his team at EGPAF Uganda are seeking to develop a model for the national scale-up of an innovative product to prevent mother-to-child
HIV transmission known as the Pratt Pouch. About 1.5 million HIV positive women give birth each year. It’s possible to prevent mother-to-child
HIV transmission if antiretrovirals (ARVs) are administered during labor and for several weeks following delivery. Unfortunately, many mothers
don’t give birth in health facilities so their infants fail to receive ARVs during this critical period, and those who do are currently sent home
with bottles of liquid ARVs and a syringe to measure appropriate doses to administer to their child. Keeping these syringes clean can prove difficult
for new moms in low-resource settings, and the viscosity of the liquid ARVs makes it challenging to measure accurate doses.
The Pratt Pouch addresses these challenges—it’s an easy to tear open package containing a premeasured dose of ARVs which can be stored safely
for up to 12 months at any temperature. Dr. Bitarakwate described the pouch as a “ketchup packet” for ARVs, noting that the pouch is an adaptation
of technology used in the food industry to package ketchup. The pouch was developed by a team of engineers at Duke University with support from
a previous Saving Lives at Birth grant. Small pilot tests of the product in individual health care settings have demonstrated promise, so Dr. Bitarakwate
is seeking funding to work with the Ugandan government to integrate the product into the country’s health system and create an effective model
for national scale-up of the product.
Reducing birth complications by increasing access to noninvasive, cloud-connected anemia screening diagnostic device
PATH
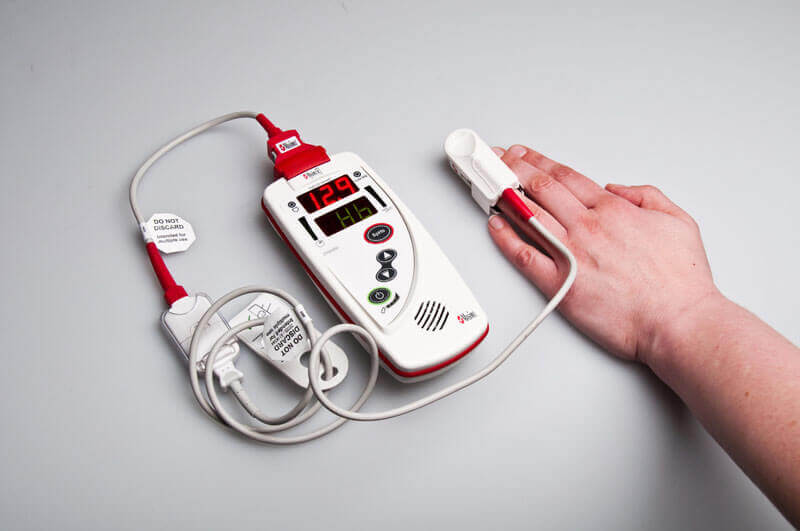
- Masimo's Pronto-7 anemia screening device. Photo: PATH/Patrick McKern
Iron-deficiency anemia affects half of all pregnant women in LMICs and can lead to poor pregnancy
outcomes including preterm births, low birth weight, and maternal mortality, contributing to one-fifth of maternal deaths. However, anemia often
goes undetected, as methods commonly used throughout sub-Saharan Africa require a blood draw, which can be prohibitive to patients and must be
administered by highly-trained health professionals. PATH consulted more than 100 nurses, doctors, and other health professionals in sub-Saharan
Africa, hearing repeatedly “noninvasive is the future” of diagnostics. PATH has partnered with California-based diagnostic manufacturer Masimo
to create a noninvasive, easy-to-use, cloud-connected diagnostic device, using the “FDA-approved guts” of Masimo’s Pronto-7 hemoglobin test. Rather than drawing blood, the health worker merely asks the patient to place their finger on the sensor (see photo) and the results
are available within a minute.
PATH will use Masimo’s equipment and technology, but will develop a device that is more durable and even easier to read—enabling use by lower-skilled
professionals. Andy Beddoe, a product development officer at PATH, explained that diagnostics that require disposable parts are more dependent
on a robust supply chain. This device, however, will require no such disposables, and will use cloud-enabled software, allowing the manufacturer
to “sell tests like you would cell phone minutes,” troubleshoot, or calibrate it remotely. Beddoe believes each device could last three to four
years, administering more than 50,000 tests. The software could also be used for data collection and could be adapted for use in other diagnostics.
A pediatric-friendly ARV dosage form: validation of palatability, flexibility, and accurate dosing of LPV/r
PATH
“[We’re] targeting the most vulnerable population infected with HIV,” explains Dr. Manjari Lal, senior research officer in Vaccine and Pharmaceutical
Technologies at PATH, in discussing her project. Less than a quarter of HIV-positive children in LMICs are receiving ARVs, and for most families, the current treatment method involves breaking adult doses into two,
crushing up one half, and mixing it with food. Lal’s team is working to transform lopinavir/ritonavir (LPV/r)—the first-line treatment for
pediatric HIV—into a product that is palatable, heat-stable, and that allows for flexible dosing. The team is developing a freeze-dried,
fast-dissolving tablet, which allows a parent to mix the medicine with water—as children often struggle with pills that must be swallowed.
According to Lal, palatability is a major consideration for pediatric treatments, as infants won’t hesitate to spit out bitter medicines. The treatment
is currently in the preclinical phase, and must go through animal studies and clinical trials with human adults before it is ready for use in children.
Scaling an Integrated Human Milk Bank and Breastfeeding promotion program in Kenya
PATH
Human milk provides important nutritional and disease-preventing properties for infants and is particularly critical for those who are born preterm
or have low birth weight. Unfortunately, some mothers are unable to produce milk or do so in the quantity needed to feed their newborn. While it’s
preferable for infants to receive their own mother’s fresh milk through breastfeeding or hand expression, in cases when that is not possible, the
World Health Organization recommends the administration of human donor milk. The idea of milk banks were developed to meet this need. These banks
accept donor milk, screen and process it for safety, and disseminate it to mothers in need. PATH was seeking a grant to scale-up and evaluate the
impact of an innovative and integrated human milking banking approach known as Mother and Baby Friendly Initiative Plus (MBFI+). Unlike
stand-alone milk banks, the MBFI+ approach creates “hubs” that provide both lactation support and milk banking at the same facility.
Kimberly Amundson—program officer on PATH’s Maternal and Child Health and Nutrition Team which is leading the project—describes MBFI+ as
a “self-reinforcing cycle of supporting breastfeeding for all infants.” Mothers come into the facilities for lactation support, and are then able
to donate any excess milk they may produce to those who need it, and those infants who receive donor milk often go on to be able to breastfeed
just fine with their own mother. The team hopes to introduce the approach at two hospitals in Kenya, evaluate its effectiveness and potential cost
savings, and position the approach for national adoption.
Clinical validation of a novel, affordable uterine balloon tamponade, developed in Africa, to reduce maternal deaths due to postpartum hemorrhage.
PATH
Postpartum hemorrhage, or severe bleeding after childbirth, is the leading cause of maternal mortality, resulting in 125,000 deaths annually. However, with just a condom and a catheter—materials readily found in health clinics—the risk can be drastically reduced.
The condom—connected to the catheter—is inserted into the uterus and slowly filled with water, inducing uterine contractions and ultimately
slowing or stopping the bleeding. While such a device, known as a uterine balloon tamponade, is readily available in high-income countries, it
can be used just once and can cost as much as $400. PATH and partners have worked to develop a device—designed and manufactured in Sou Africa with input from health professionals—that costs just a few dollars. While condoms work well, they vary greatly and the thickness of
a condom can impact the pressure exerted by the device. This uterine balloon tamponade involves a reusable bag that is filled with water and held
at a certain height—depending on how much pressure is needed—connected to a reusable catheter which is in turn connected to the disposable,
standardized balloon. The device has been successfully tested and with widespread introduction, an estimated 169,000 deaths could be prevented over the next fifteen years. PATH was seeking funding to conduct a clinical validation study of the product and develop a commercialization strategy
to increase access to the product in LMICs.
Use of a low-cost simulation model to expand postpartum intrauterine device services
Jhpiego

- The Mama-U simulator at the Jhpiego display. Photo: GHTC/Marissa Chmiola
Over 90,000 maternal deaths can be prevented each year through family planning use. Yet, many women who have just given birth don’t receive access
to long-acting family planning methods like intrauterine contraceptive devices (IUCDs) because there are not enough health providers trained to
properly administer IUCDs to women with postpartum bodies; and many women incorrectly believe the product may not stay in place given the changes
to their bodies resulting from birth. Under a previous grant, Jhpiego found that using a postpartum body simulator called the Mama-U was effective
for training workers and improving service delivery. According to Dr. Fauzia Assad, a medical doctor working with Jhpiego Pakistan, the Mama-U
offers benefits over existing approaches to training for postpartum IUCD training. “It’s cost-effective, just $50 versus $600 for the usual model,
culturally appropriate, easy to take along, very portable, easy to understand, and it offers the same level of difficulty one would experience
with an actual patient because it has a similar feel and tension.” In earlier testing, Jhpiego found the product was not only helpful in training
providers, but counselors found it helpful in educating patients, dispelling patient concerns, and increasing client use of IUCDs. Jhpiego was
seeking a grant to build on past learning and show that the Mama-U can improve quality of services and patient uptake and change the way postpartum
IUCD training, counseling, and services are delivered.
Projecting Health India
PATH
People typically find it easier to learn a process if they see it demonstrated, rather than just hearing or reading about it. This is why the Projecting
Health India program is using video as a medium through which to promote health behaviors. Projecting Health India a community-based, video education
project, which trains communities in India to create and produce videos addressing a variety of health topics—including birth preparedness,
breastfeeding, and family planning—which are shown at community mothers’ groups and village health days. The videos feature local actors
and health workers and aren’t distributed outside the district for which they were produced. Michelle Desmond, a monitoring and evaluation officer
at PATH involved with the program, described the approach “as hyperlocal” which is key to the program’s effectiveness. “Literature on behavior
change shows that if you can empathize with a person you see practicing a healthy behavior, you are more likely to practice it yourself,” said
Desmond. “So in these videos women are seeing people who sound like them, who look like them, who are in the same situation as them practicing
these health behaviors.” After realizing that the videos were so popular that women were finding ways to transfer them onto their mobile phones
to rewatch and share, Projecting Health partnered with mobile phone vendors in India to allow the videos to be easily loaded onto phones, providing
an additional way for these videos to be distributed to harder to reach mothers. PATH was seeking a Saving Lives at Birth grant to scale up this
project in government systems throughout the state of Uttar Pradesh.
NIFTY: A Neonatal Intuitive Feeding Technology for preterm infants who have difficulty breastfeeding
PATH

- A demonstration of feeding using the NIFTY cup. Photo: GHTC/Marissa Chmiola
In high-resource settings, infants who struggle with breastfeeding can be fed with breast milk through tubes or bottles,
however such technologies can be expensive, difficult-to-use, or unsanitary in low-resource settings. In LMICs, the World Health Organization recommends
the use of small cups for infants with feeding difficulties, including preterm infants, infants who have lost their mother due to complications
in pregnancy or childbirth, and children with cleft palates. There is currently no product on the market specifically designed for this purpose,
and the metal or glass cups that are often used can be inefficient, or can cause injury to the infant’s mouth including cuts. To address these
limitations, PATH and its partners created the NIFTY™ cup which is made of silicone, making it soft and durable. The cup can also be boiled
without causing any damage—making it easy to sterilize. The cup also has a reservoir on the lip of the cup, making it easy to control the
amount of milk delivered and prevent waste. The NIFTY™ cup costs just $1.25 to produce. This innovation was chosen as one of the 17 competition
award nominees. The team at PATH will use the funding to test the NIFTY™ cup in hospital settings in Ethiopia and work with private-sector
partners throughout the sub-Saharan market to commercialize the cup.
Innovation is happening around the world
In visiting the DevelopmentXChange, it was clear that innovation is happening in all corners of the globe and lifesaving ideas can come from anywhere
and anyone. Saving Lives at Birth demonstrates the power of bringing governments, foundations, academic institutions, and for-profit companies
together to address our most pressing health challenges. GHTC congratulates both our members and the other teams who were selected as finalists
and award nominees—your work continues to inspire us.
Authored by Marissa Chmiola, GHTC’s communications officer with support from Courtney Carson, senior policy and advocacy associate, and Kat Kelley, senior program assistant.






