Cherise ScottTB Alliance
Cherise Scott is Director of the Pediatric Programs at TB Alliance, a not-for-profit organization dedicated to finding faster-acting and affordable drug regimens to fight tuberculosis.

Early this month, GHTC member TB Alliance and its partners announced the creation of the first-ever appropriate, child-friendly tuberculosis (TB) medicine in the correct doses. These treatments, which will be available in early 2016, are simple to administer, affordable, and the first to meet childhood TB dosage treatment guidelines set by the World Health Organization (WHO) in 2010.
GHTC’s Breakthroughs blog is joined by TB Alliance’s Cherise Scott, Director of the Pediatric Programs and Willo Brock, Senior Vice President, to learn more about this exciting development that could dramatically improve treatment for the one million children with TB each year.
Q: Why do we need TB medicines designed specifically for children? And how are these new treatments more child-friendly than what’s in use today?
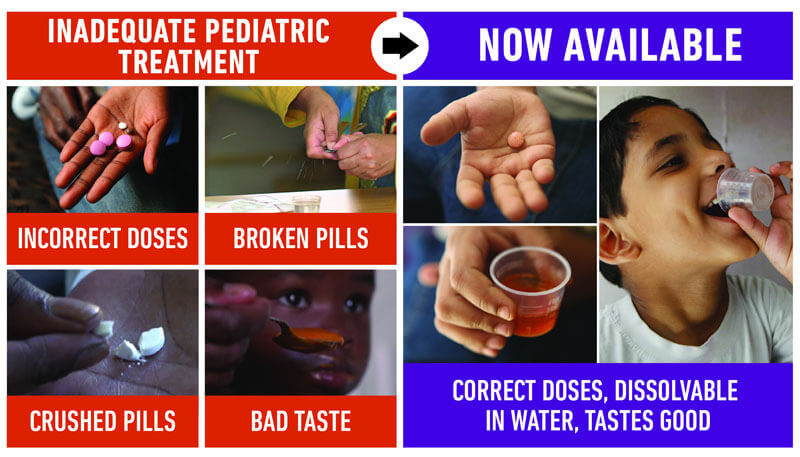
Children need different dosing of their TB treatment than their parents. Until now, this meant that parents needed to split or crush pills to get to the right dosage, which is difficult. The current pills also taste bad, which can lead to children refusing to take their medicine and thus poor adherence and growth in drug resistance. The new formulations that are available are appropriately dosed, dissolvable, and fruit-flavored, which will improve adherence. This should simplify and improve treatment for children everywhere. For more information on what these medicines mean for children with TB and their families, see this video.
Q: According to WHO, 140,000 children die each year from TB. Given this tremendous need, why did it take so long for appropriately dosed, child-friendly TB medicines to be developed?
Not only do 140,000 children die, at least one million children get sick every year from TB. However, children with TB have been neglected for too long. Children with TB often live in poverty, and companies therefore do not have a commercial incentive to invest in research and development for improved TB treatments. After WHO created specific dosage guidelines for children in 2010, TB Alliance, with WHO and a range of other partners, worked together with funding from UNITAID, the US Agency for International Development, and others to advance product development and introduce important medicines that fulfill this critical gap.
Q: In product development we know that successfully developing a new product is only half the battle; it needs to actually reach patients to have an impact. What’s next for these treatments in terms of regulatory approval, introduction, and product roll-out?

In early December 2015, TB Alliance announced the availability of the products under WHO’s “ERP” [Expert Review Panel] mechanism, which is an interim step to expedite access to lifesaving drugs. That means the products are now available for procurement through the Stop TB Partnership’s Global Drug Facility. Full WHO Prequalification is expected in 2016, which will enable even greater access. However, various countries require additional steps to register the treatments. We are asking everyone to help accelerate access to appropriate TB treatments for children by signing our call to action and asking governments to speed up approval and adoption of the new child-friendly treatment. Please sign it and spread the word!