Kat KelleyGHTC
Kat Kelly is a senior program assistant at GHTC who supports GHTC's communications and member engagement activities.
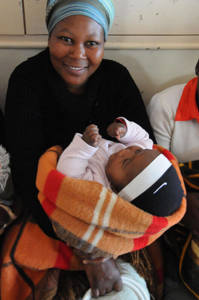
Many of the 15 million babies born premature each year struggle with hypothermia and need help to stay warm. To address this problem, researchers at Johns Hopkins University and George Mason University developed a thermal blanket that uses the chemical substance found in hand warmers and pain patches. The International Centre for Diarrhoeal Disease Research, Bangladesh (icddr,b) is currently testing the blanket to determine its acceptability and feasibility among mothers and health care providers in Bangladesh and initial results suggest that it is easy-to-use, portable, and can be affordably produced using local materials. Next, the team will incorporate feedback on the blanket’s design and use from the study and a stakeholder’s workshop and will test the efficacy of the blanket in reducing newborn mortality.
In India, where there are only seven doctors for every 10,000 people—compared to a global average of 13.9—and where nearly six times as many critical care doctors are needed, electronic intensive care units (eICUs) are expanding access to quality, intensive care. In these eICUs, critical care specialists monitor patients and their vital signs and as needed, provide instructions to local physicians—who may not have any intensive care experience—remotely. Across the country, there are currently 3,000 eICU “beds,” and a 15–20 percent is expected annually. Fortis Healthcare, a chain of hospitals that currently monitors 350 patients, estimates that they save 25 lives per month. While the cost of monitoring patients remotely is only US$10–30 per day, the standard cost of critical care within the hospital ($200 per day) remains, and thus, access to the technology remains limited to those who can afford it.
The United States, European Union, and World Health Organization (WHO) are seeking to expedite Zika virus research and development (R&D) through financing and innovative regulatory mechanisms. The White House is calling on the US Congress to provide $1.8 billion in funding to combat Zika, including $200 million specifically for vaccine and diagnostic R&D. The request also includes funding for the US Agency for International Development to incentivize R&D through public-private partnerships, advanced market commitments, or volume guarantees. Meanwhile, legislation has been introduced in both the House and Senate to add Zika virus to the list of diseases eligible for the US Food and Drug Administration’s Priority Review Voucher program, through which a pharmaceutical company is granted a voucher (worth up to $350 million) for expedited review of a future drug as a reward for successfully sponsoring the review of a drug for a neglected disease.
Meanwhile, the European Medicines Agency has established a task force to advise on scientific and regulatory issues—including clinical trial design, manufacturing, and post-market surveillance—for tools to prevent, detect, and treat Zika. The European Commission is offering $10 million for Zika vaccine R&D, using existing funding for vaccine development through Horizon 2020, which promotes research and innovation across sectors. The WHO plans to use its Emergency Use Assessment and Listing procedures to rapidly evaluate Zika products for quality, efficacy, and safety. This mechanism is particularly invaluable as United Nations agencies and many nongovernmental organizations rely on WHO evaluations when making procurement decisions.